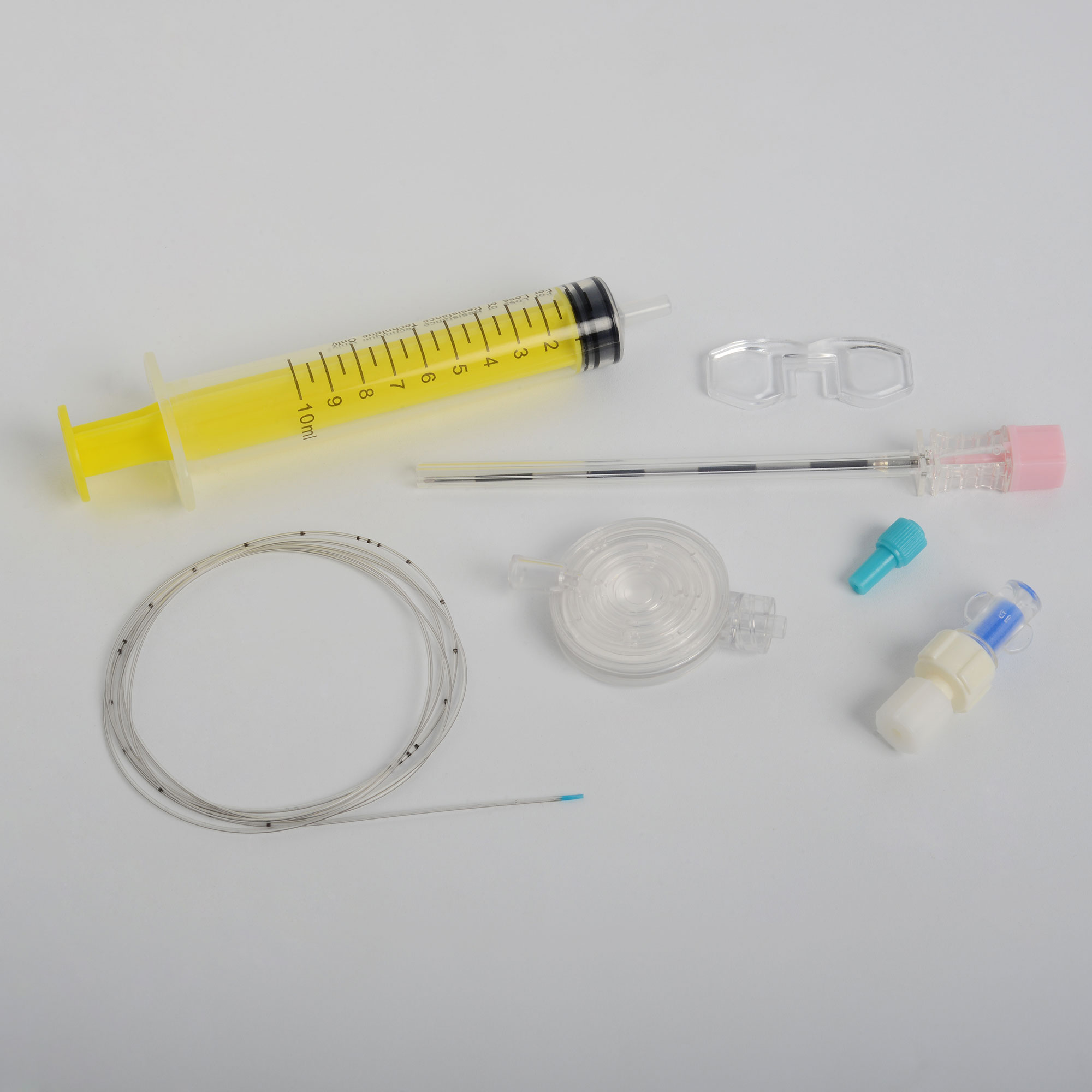
Neuraxial devices, such as epidural catheters, Needle, & Spinal Needles are used to deliver medicines or anesthesia to neuraxial sites, such as the epidural space, or are used to monitor or remove cerebral-spinal fluid for therapeutic or diagnostic purposes. ISO 80369-6:2016, was published in March 2016, to provide specifications for designing the connectors for use with neuraxial devices. Medzus Medical recognizes this standard.
NRfit is designed to ensure that neuraxial device connectors are incompatible with the connectors for unrelated delivery systems such as IV lines, and other vascular catheters. Misconnections involving such medical devices may be relatively rare compared with the number of patients needing tubes or IVs, but such misconnections can have deadly consequences when they do occur.
Medzus Medical is committed to the prevention of misconnections and actively supports the ongoing worldwide patient safety initiatives dedicated to reducing tubing misconnections by supplying following ISO 80369-6 compliance devices.