Reducing the Risk associated with small bore connector medical device tubing misconnections & to improves the Patient Safety
ISO 80369 Small Bore Connectors
Our Moto
What is small bore Connectors?
Many different types of medical devices incorporate small-bore connectors. Small-bore connectors are parts used to connect medical devices such as tubing, syringes, and other accessories that deliver fluids and gases for patient care. Small-bore refers to the small size of the diameter opening (less than 8.5 millimeters) of the connector.
What is Medical Device Misconnection?
Medical device misconnections may occur when one type of medical device is mistakenly attached to another type of medical device that performs a different function. Because these connectors are easy to use and may be compatible with different medical devices, users can mistakenly connect unrelated systems to one another. This may cause medication or other substances to be delivered through the wrong tubing into the incorrect area of the body. These errors are sometimes called tubing misconnections, wrong route errors, catheter misconnections, or Luer misconnections and can result in patient injury or death.
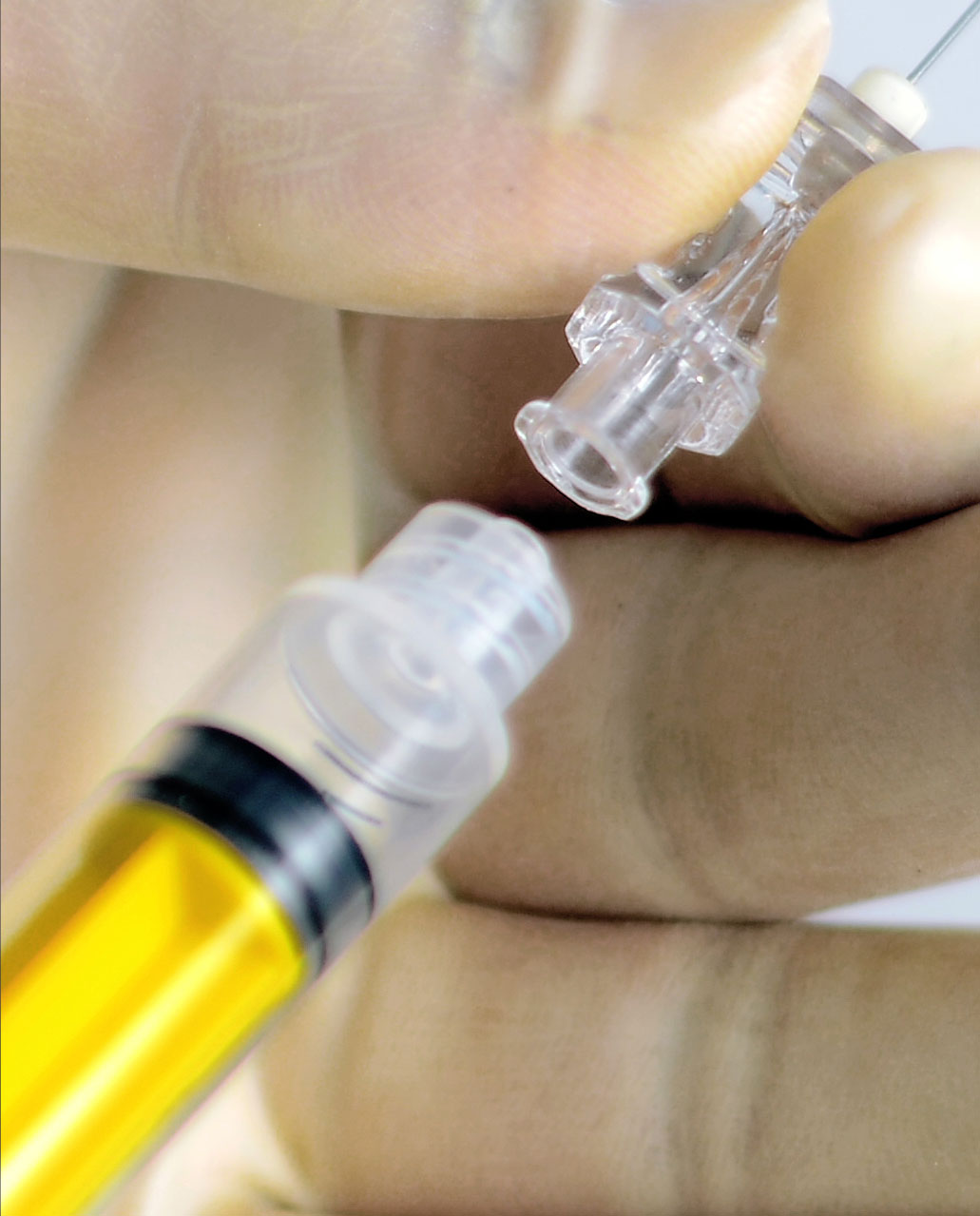
Device misconnections can occur for many reasons, including:
- The current similar design of many connectors and widespread use of connectors with similar sizes and shapes.
- Human error, arising from conditions such as multiple connections on one patient, poor lighting, lack of training, time pressure, fatigue or high-stress environments.
Manufacturers and health care facilities have tried many methods to prevent device misconnections, including color-coding, labels, tags, and training. However, these methods alone have not effectively solved the misconnection problem because they are not consistently applied, nor do these methods physically prevent the misconnections.
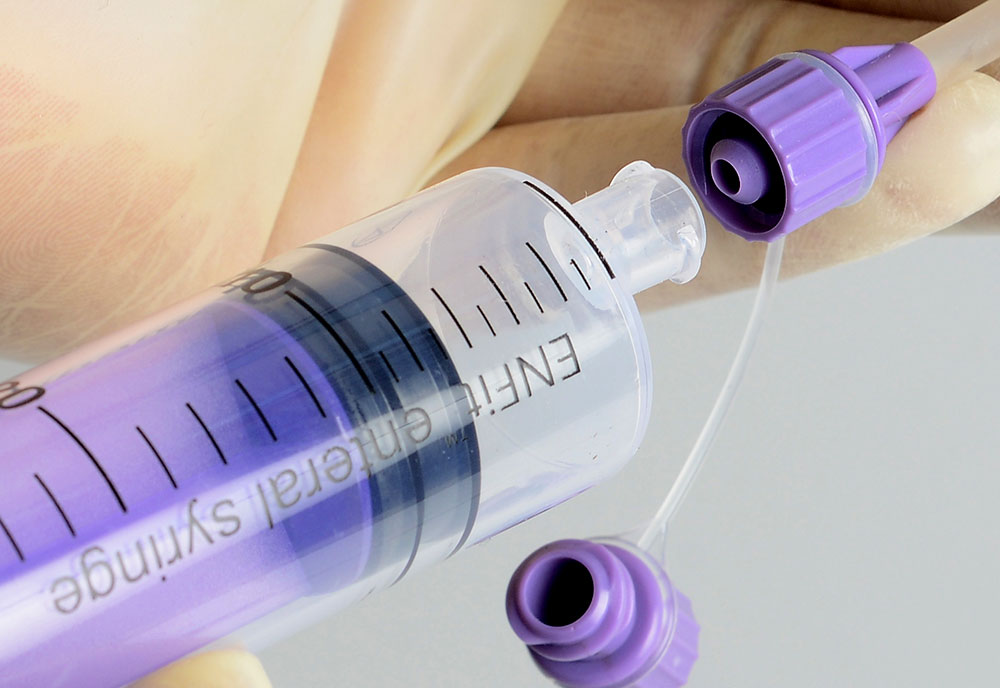
How Reducing the Risk of Misconnection?
The way devices connect to each other are changing, greatly reducing the risk for misconnections. New design standards are being developed for tubing connectors for high-risk medical applications (e.g., enteral, respiratory, neuraxial), so that unrelated devices cannot connect with each other.
Hence, Medzus Medical is committed to the prevention of misconnections and actively supports the ongoing worldwide patient safety initiatives dedicated to reducing tubing misconnections by supplying ISO 80369 compliance devices.
Small bore connectors are being defined with appropriate anatomical location & are categorized to use for specific application with unique geometrical design. These new connectors are being designed to avoid misconnection.
